INSIGHT: LENS trial newsletter no. 5, June 2024
Welcome to the fifth edition of INSIGHT, the newsletter of the LENS Trial. We are very happy to be able to share the final results for the trial with you. This newsletter also explains how you can find out if you were receiving fenofibrate or placebo during the trial, if you are interested to know.
Than you very much for supporting the trial.
Associate Professor David Preiss and the LENS team.
LENS results: Fenofibrate reduces the risk of worsening retinopathy
The trial was designed to find out whether treatment with fenofibrate, for about four years, might reduce the chance of people with mild to moderate diabetic retinopathy needing treatment (with laser, eye injections or a vitrectomy operation) or needing to be referred to a specialist due to retinopathy.
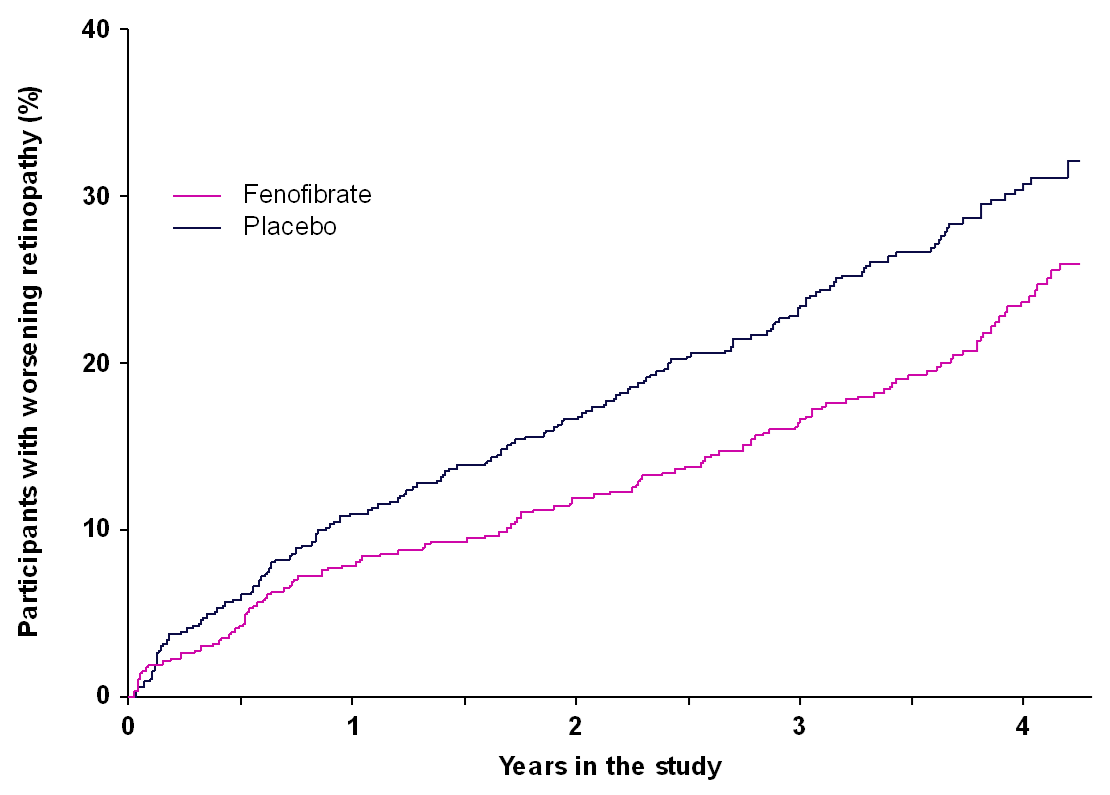 We are delighted to report that the trial showed positive results. Overall, 168 (29.2%) of 575 participants on placebo had worsening retinopathy compared to 131 (22.7%) of 576 participants on fenofibrate, a reduction of 6.5%. In other words, for every 100 participants receiving placebo who developed worsening retinopathy during the trial, 73 participants receiving fenofibrate developed worsening retinopathy but 27 participants were protected from worsening retinopathy. The image on the left shows the results from LENS — the black line represents participants receiving placebo with worsening retinopathy and the pink line represents those receiving fenofibrate. You can see how the lines separated over time. We also found that fewer people receiving fenofibrate developed macular oedema (swelling in the retina) than those receiving placebo.
We are delighted to report that the trial showed positive results. Overall, 168 (29.2%) of 575 participants on placebo had worsening retinopathy compared to 131 (22.7%) of 576 participants on fenofibrate, a reduction of 6.5%. In other words, for every 100 participants receiving placebo who developed worsening retinopathy during the trial, 73 participants receiving fenofibrate developed worsening retinopathy but 27 participants were protected from worsening retinopathy. The image on the left shows the results from LENS — the black line represents participants receiving placebo with worsening retinopathy and the pink line represents those receiving fenofibrate. You can see how the lines separated over time. We also found that fewer people receiving fenofibrate developed macular oedema (swelling in the retina) than those receiving placebo.
The results were presented at the American Diabetes Association’s annual meeting on Friday 21st June 2024.
future plans for the lens trial
We believe that the LENS trial has produced important results that support the use of fenofibrate to treat people with diabetes who have mild to moderate retinopathy. However, treatment with fenofibrate is unlikely to be offered routinely in the NHS until diabetes guidelines are updated to recommend using it.
The LENS investigators will continue our research to better understand the effect of fenofibrate in the eye. When you signed up to join LENS, you gave consent for us to track your NHS Scotland records for up to 10 years after the trial. Given the positive results of the trial, this work is now even more important. We therefore hope that you are happy for this NHS ‘data linkage’ work to continue. However, if you do not want your records to be used in this way, please let us know (using the telephone number below).
If you wish to know whether you received fenofibrate or placebo, you can also call us (weekdays 9AM-5PM).
opportunities to contribute towards other research
The LENS team is keen to promote opportunities for people to join other research studies. We therefore wanted to let you know about SHARE.
SHARE is an NHS Research Scotland initiative to establish a register of people, aged 11 and over, who are interested in participating in health research in Scotland. Participants agree to allow SHARE to use the coded data in their various secure NHS computer records to check whether they might be suitable to participate in various health research studies. All information is confidential. No-one can access this information without your permission. This information can be very useful when it comes to developing new treatments and cures for a wide variety of health conditions. You can read stories from those who have already signed up on the website https://www.registerforshare.org/why-i-shared.
If you are interested to read more about SHARE, you can visit the website https://www.registerforshare.org/
Participants in SHARE can also volunteer to allow any spare blood, which is left over following routine NHS clinical tests, to be used for anonymised health research. This is known as the SHARE Biobank. As just one example of the potential benefits of this approach, if you join the SHARE Biobank then the LENS research team may be able to use the information obtained from your spare blood to better understand how fenofibrate helps reduce the risk of diabetic retinopathy becoming worse.
thank you for your contribution to the lens trial!
Freefone: 0808 164 5090 lens@ndph.ox.ac.uk www.ctsu.ox.ac.uk/lens



